Vancouver Style: The University of Queensland have a modified Vancouver Style on their Library website
More than 6000 styles are available for download from the EndNote Website.
How to install additional styles
1. Download the style (for JLSB reference style click here)
2. Double-click the style file. It should open in EndNote
3. On the open style, go to “File Menu” and choose “Save As”. Replace the word “copy” with your style’s name and click “Save”.
4. Click on “File Menu” and choose “Close Style”
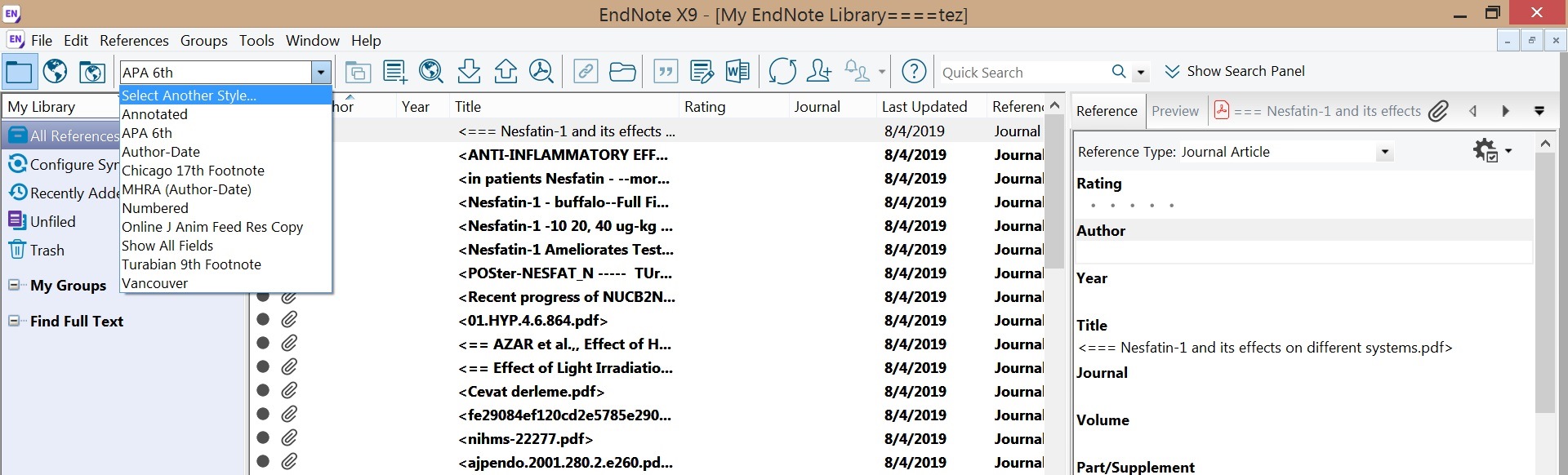
5. Then Go back to your EndNote Library and go to "select another style". Choose it from the list
Where to save your styles
The 400 styles which come with EndNote are usually located in this location:
These can stay here
C:\Program Files\EndNote\Styles
Any extra styles will need to be saved outside of your Program files, into your personal styles folder. EndNote will then merge these folders into one list when you go to choose a style.
To find the correct folder location for your personal styles folder, first open EndNote and look in Edit>Preferences>Folder locations. This will tell you the default file path. The first time you should create the Styles folder following this pattern.
It will look similar to this:
C:\Users\UserName\My Documents\EndNote\Styles
Save any modified styles you download into your personal styles folder
Change Styles
In EndNote, reference or bibliographic style templates are called 'Output Styles'. EndNote supports output styles for in-text citations, numbered in-text citations, and footnotes.
Approx 500 common styles come pre-loaded with EndNote, listed under "Select Another Style".
Choose a Style
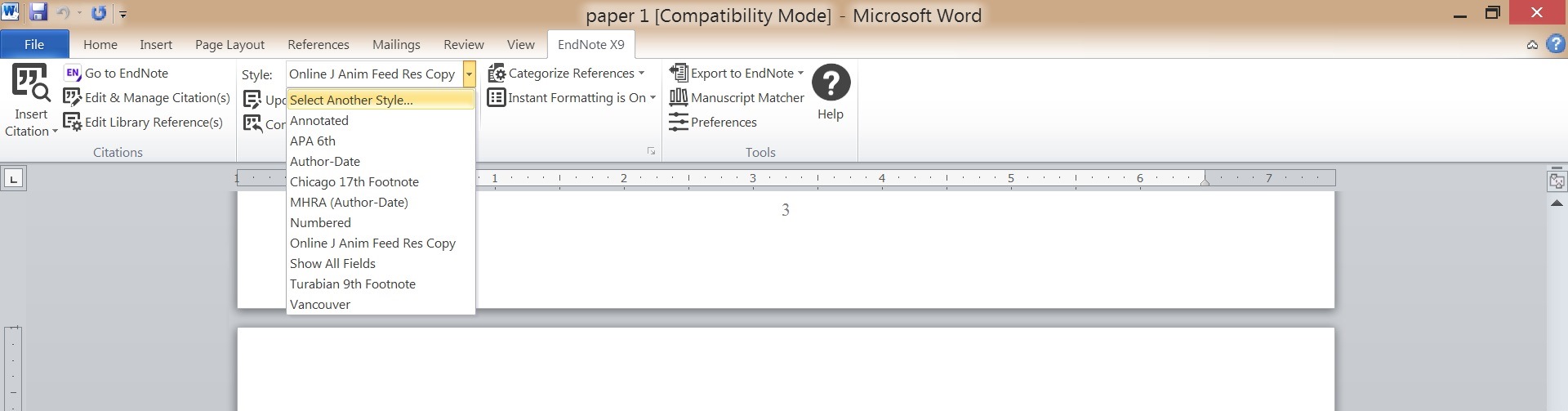
Change Styles in Word
Go to the EndNote Toolbar in Word to change styles and update citations/bibliography in your Word document
Can't find your style in the list?
If the style you just installed does not appear in the drop-down styles menu in EndNote or Word, you need to activate the style by:
- Clicking on Edit in the top toolbar
- Select Styles > Open style manager
- Find and tick the box for the styles you need
- Close the Style manager
- Close and re-open EndNote
- This style will now be available for you to choose
Graphical Abstract
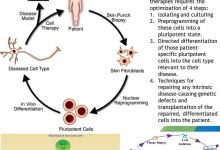
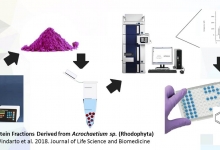
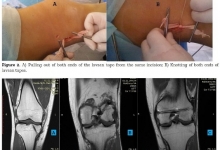
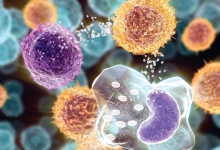
A Graphical Abstract is a single, concise, pictorial and visual summary of the main findings of the article. This could either be the concluding figure from the article or a figure that is specially designed for the purpose, which captures the content of the article for readers at a single glance. Please see the attached examples.
The Graphical Abstract will be displayed in online search result lists, the online contents list and the online article, but will not (yet) appear in the article PDF file or print.
Author instructions
A Graphical Abstract should allow readers to quickly gain an understanding of the main take-home message of the paper and is intended to encourage browsing, promote interdisciplinary scholarship, and help readers identify more quickly which papers are most relevant to their research interests. Authors must provide an image that clearly represents the work described in the paper. A key figure from the original paper, summarising the content can also be submitted as a graphical abstract.
Specifications
A Graphical Abstract should be a one-image file and should visualize one process or make one point clear. For ease of browsing, the Graphical Abstract should have a clear start and end, preferably "reading" from top to bottom or left to right. Try to reduce distracting and cluttering elements as much as possible.
- Image size: Please provide an image with a minimum of 500 x 1300 pixels (hxw) using a minimum resolution of 300 dpi. If you are submitting a larger image then please use the same ratio (200 high x 500 wide).
- Font: Please use Times, Arial, Courier or Symbol font with a large enough font size as the image will be reduced in size for the Table of Contents to fit a window of 200 pixels high.
- File type: preferred file types are TIFF, EPS, PDF or MS Office files.
- No additional text, outline or synopsis should be included. Any text or label must be part of the image file. Please do not use unnecessary white space or a heading “Graphical Abstract” within the image file.
See GA examples
| JLSB EndNote Style |
| Manuscript Template (.doc) |
| Sample Articles |
| Declaration form |
| Policies and Publication Ethics |
Guide for Authors
.
Table of Contents:
PREPARATION OF MANUSCRIPT
Article sections format
Declarations
REFERENCES
Review/Decisions/Processing
.
Manuscript as Original Research Paper, Short Communication, Case Reports and Review or Mini-Review are invited for peer-review publishing in the Journal of Life Science and Biomedicine (JLSB). Considered scientific areas include: cell biology, biochemistry, molecular biology, genetics, immunology, pathology, virology, microbiology, parasitology, epidemiology, pharmacology, biophysics, biopsychology, neuroscience, psychopharmacology, nanobiotechnology, cancer biology, surgery, bioinformatics and computational biology, zoonotic diseases, public health, vaccination, case reports of rare diseases, zoology, veterinary, ecosystems and biodiversity, populations, human evolution and behavior, air and soil pollution and biological control, environmental microbiology, biomass and biopower, marine ecology, conservation and evolutionary biology.
The Journal of Life Science and Biomedicine publishes all accepted articles every quarter (four times a year).
.
Submission
The manuscript and other correspondence should be submit online preferentially. Please embed all figures and tables in the manuscript to become one single file for submission. Once submission is complete, the system will generate and sent a manuscript ID and the confirmation email to the corresponding author's contact email: This email address is being protected from spambots. You need JavaScript enabled to view it.. All manuscripts must be checked (by English native speaker) and submitted in English for evaluation (in totally confidential and impartial way).
Supplementary information
The online submission form allows supplementary information to be submitted together with the main manuscript file and covering letter. If you have more than one supplementary files, you can submit the extra ones by email after the initial submission. Author guidelines are specific for each journal. Our Word template can assist you by modifying your page layout, text formatting, headings, title page, image placement, and citations/references such that they agree with the guidelines of journal. If you believe your article is fully edited per journal style, please use our Word template before submission.
Supplementary materials may include figures, tables, methods, videos, and other materials. They are available online linked to the original published article. Supplementary tables and figures should be labeled with a "S", e.g. "Table S1" and "Figure S1". The maximum file size for supplementary materials is 10MB each. Please keep the files as small possible, to avoid the frustrations experienced by readers with downloading large files.
.
Submission to the journal is on the understanding that
1. The article has not been previously published in any other form and is not under consideration for publication elsewhere;
2. All authors have approved the submission and have obtained permission for publish work.
3. Researchers should comply with the ARRIVE guidelines (Animal Research: Reporting of In Vivo Experiments), and also the WMA Declaration of Helsinki (Ethical Principles for Medical Research Involving Human Participants).
Ethics Committee Approval
Experimental research involving human or animals should have been approved by author's institutional review board or ethics committee. This information can be mentioned in the manuscript including the name of the board/committee that gave the approval, the protocol no. and the approval date. Investigations involving humans will have been performed in accordance with the WMA Declaration of Helsinki – Ethical Principles for Medical Research Involving Human Participants. And the use of animals in experiments should be complied with the ARRIVE guidelines (Animal Research: Reporting of In Vivo Experiments) and also the Interdisciplinary Principles and Guidelines for the Use of Animals in Research, Testing, and Education by the New York Academy of Sciences, Ad Hoc Animal Research Committee.
Clinical trials
The JLSB follow the the International Committee of Medical Journal Editors (ICMJE) Recommendations for the Conduct, Reporting, Editing and Publication of Scholarly Work in Medical Journals. Hence submitted manuscripts are expected to comply with the ICMJE Recommendations including Roles & Responsibilities, Publishing & Editorial Issues, Manuscript Preparation and Submission. View additional duties and responsibilities that are summarized at Publication ethics and Policies.
.
Patient’s confidentiality
Authors of clinical papers are obliged to carefully protect patient's identities and privacy rights. If the manuscript contains photos of patients, informed consent from each patient should be obtained prior to publication. Only clinically or scientifically important data are permitted for publishing.
.
Competing Interests
Competing interests that might interfere with the objective presentation of the research findings contained in the manuscript should be declared in a paragraph heading "Competing interests" (after Acknowledgment section and before References). Examples of competing interests are ownership of stock in a company, commercial grants, board membership, etc. If there is no competing interest, please use the statement "The authors have declared that no competing interest exists.
.
Graphical Abstract
Authors should provide a graphical abstract (a beautifully designed feature figure) to represent the paperaiming to catch the attention and interest of readers. Graphical abstract will be published online in the table of content.The graphical abstract should be colored, and kept within an area of 12 cm (width) x 6 cm (height) or with similar format. Image should have a minimum resolution of 300 dpi and line art 1200dpi.
Note: Height of the image is generally no more than the width. Authors should avoid putting too much information into the graphical abstract as it occupies only a small space. The graphical abstract can be presented in the format of PowerPoint, Word, PDF, JPG, PNG or TIFF, after a manuscript is accepted for publication. For preparing a Professional Graphical Abstract, please click here.
.
Main format
First page of the manuscripts must be properly identified by the title and the name(s) of the author(s). It should be typed in Times New Roman (font sizes: 17pt in capitalization for the title, 10pt for the section headings in the body of the text and the main text, 9pt for References, double spaced, in A4 format with 2cm margins. All pages and lines of the main text should be numbered consecutively throughout the manuscript. The manuscript must be saved in a .doc format, (not .docx files). Abbreviations in the article title are not allowed.
.
Manuscripts should be arranged in the following order:
a. TITLE (brief, attractive and targeted);
b. Name(s) and Affiliation(s) of author(s) (including post code and ORCID ID) and corresponding author's E-mail;
c. ABSTRACT;
d. Keywords (separate by semicolons; or comma,);
e. Abbreviations (used in the manuscript);
f. INTRODUCTION;
g. MATERIALS AND METHODS;
h. RESULTS;
i. DISCUSSION;
j. CONCLUSION;
k. DECLARATIONS (Acknowledgements, Authors' contributions, Competing interests, etc.)
1. REFERENCES;
m. Tables;
n. Figure captions;
o. Figures;
Results and Discussion can be presented jointly if preferred.
Discussion and Conclusion can be presented jointly if preferred.
Large Language Models (LLMs), such as ChatGPT, do not meet our authorship criteria. Notably an attribution of authorship carries with it accountability for the work, which cannot be effectively applied to LLMs.
.
Title should be a brief phrase describing the contents of the paper. The first letter of each word in the title should use upper case. The Title Page should include the author(s)'s full names and affiliations, the name of the corresponding author along with phone and email information. Present address (es) of the author(s) should appear as a footnote.
.
Abstract should be informative and completely self-explanatory, briefly present the topic, state the scope of the experiments, indicate significant data, and point out major findings and conclusions. The abstract should be 150 to 350 words in length. Complete sentences, active verbs, and the third person should be used, and the abstract should be written in the past tense. Standard nomenclature should be used and abbreviations should be avoided. No literature should be cited.
.
Following the abstract, about 3 to 8 keywords that will provide indexing references should be listed. In order to assist indexers in cross-indexing articles please use keywords from the Medical Subject Headings (MeSH) list of Index Medicus (http://www.nlm.nih.gov/mesh/MBrowser.html).
.
Introduction should provide a clear statement of the problem, the relevant literature on the subject, and the proposed approach or solution. It should be understandable to colleagues from a broad range of scientific disciplines.
.
Material and Method should be complete enough to allow experiments to be reproduced. However, only truly new procedures should be described in detail; previously published procedures should be cited, and important modifications of published procedures should be mentioned briefly. Capitalize trade names and include the manufacturer's name and address. Subheadings should be used. Methods in general use need not be described in detail. The ethical approval for using humans and animals in the research should be indicated in this section or Declarations section with a separate title.
.
Results should be presented with clarity and precision. The results should be written in the past tense when describing findings in the author(s)'s experiments. Previously published findings should be written in the past tense. Results should be explained, but largely without referring to the literature. In case of the effectiveness of a particular drug or other substances as inhibitor in biological or biochemical processes, the results should be provided as IC50 (half maximal inhibitory concentration) or similar appropriate manner. Discussion, speculation and detailed interpretation of data should not be included in the results but should be put into the discussion section.
.
Discussion should interpret the findings in view of the results obtained in this and in past studies on this topic. State the conclusions in a few sentences at the end of the paper. The Results and Discussion sections can include subheadings, and when appropriate, both sections can be combined.
.
Conclusion should be brief and tight about the importance of the work or suggest the potential applications and extensions. This section should not be similar to the Abstract content.
.
Declarations including Acknowledgements, Author contribution, Competing interests, Consent to publish, and Availability of data etc.
.
Tables should be kept to a minimum and be designed to be as simple as possible. Tables are to be typed double-spaced throughout, including headings and footnotes. Each table should be on a separate page, numbered consecutively in Arabic numerals and supplied with a heading and a legend. Tables should be self-explanatory without reference to the text. The details of the methods used in the experiments should preferably be described in the legend instead of in the text. The same data should not be presented in both table and graph forms or repeated in the text.
.
Figure legends should be typed in numerical order on a separate sheet. Graphics should be prepared using applications capable of generating high resolution GIF, TIFF, JPEG or PowerPoint before pasting in the Microsoft Word manuscript file. Use Arabic numerals to designate figures and upper case letters for their parts (Figure 1). Begin each legend with a title and include sufficient description so that the figure is understandable without reading the text of the manuscript. Information given in legends should not be repeated in the text.
Declarations section
Please ensure that the sections:
- Acknowledgements
- Authors' contribution
- Availability of data and materials
- Competing interests
- Ethics (and consent to participate)
are included at the end of your manuscript in a Declarations section.
.
Acknowledgements
We strongly encourage you to include an acknowledgements section between the Authors’ contributions section and Reference list. Please acknowledge anyone who contributed towards the study by making substantial contributions to conception, design, acquisition of data, or analysis and interpretation of data, or who was involved in drafting the manuscript or revising it critically for important intellectual content, but who does not meet the criteria for authorship. Please also include their source(s) of funding. Please also acknowledge anyone who contributed materials essential for the study.
Authors should obtain permission to acknowledge from all those mentioned in the Acknowledgements. Please list the source(s) of funding for the study, for each author, and for the manuscript preparation in the acknowledgements section. Authors must describe the role of the funding body, if any, in study design; in the collection, analysis, and interpretation of data; in the writing of the manuscript; and in the decision to submit the manuscript for publication.
.
Authors’ contribution
For manuscripts with more than one author, JLSB require an Authors' Contributions section to be placed after the Competing Interests section.
An 'author' is generally considered to be someone who has made substantive intellectual contributions to a published study. To qualify as an author one should 1) have made substantial contributions to conception and design, or acquisition of data, or analysis and interpretation of data; 2) have been involved in drafting the manuscript or revising it critically for important intellectual content; and 3) have given final approval of the version to be published. Each author should have participated sufficiently in the work to take public responsibility for appropriate portions of the content. Acquisition of funding, collection of data, or general supervision of the research group, alone, does not justify authorship.
We suggest the following format (please use initials to refer to each author's contribution): AB carried out the molecular genetic studies, participated in the sequence alignment and drafted the manuscript. JY carried out the immunoassays. MT participated in the sequence alignment. ES participated in the design of the study and performed the statistical analysis. FG conceived of the study, and participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
For authors that equally participated in a study please write 'All/Both authors contributed equally to this work.'
Contributors who do not meet the criteria for authorship should be listed in an acknowledgements section.
.
Competing interests
Competing interests that might interfere with the objective presentation of the research findings contained in the manuscript should be declared in a paragraph heading "Competing interests" (after Acknowledgment section and before References). Examples of competing interests are ownership of stock in a company, commercial grants, board membership, etc. If there is no competing interest, please use the statement "The authors declare that they have no competing interests.".
Journal of Life Science and Biomedicine adheres to the definition of authorship set up by The International Committee of Medical Journal Editors (ICMJE). According to the ICMJE authorship criteria should be based on 1) substantial contributions to conception and design of, or acquisition of data or analysis and interpretation of data, 2) drafting the article or revising it critically for important intellectual content and 3) final approval of the version to be published. Authors should meet conditions 1, 2 and 3. It is a requirement that all authors have been accredited as appropriate upon submission of the manuscript. Contributors who do not qualify as authors should be mentioned under Acknowledgements.
.
Consent to publish
Please include a ‘Consent for publication section in your manuscript. If your manuscript contains any individual person’s data in any form (including individual details, images or videos), consent to publish must be obtained from that person, or in the case of children, their parent or legal guardian. All presentations of case reports must have consent to publish. You can use your institutional consent form or our consent form if you prefer. You should not send the form to us on submission, but we may request to see a copy at any stage (including after publication). If your manuscript does not contain any individual persons data, please state “Not applicable” in this section.
.
Change in authorship
We do not allow any change in authorship after provisional acceptance. We cannot allow any addition, deletion or change in sequence of author name. We have this policy to prevent the fraud.
.
Data deposition
Nucleic acid sequences, protein sequences, and atomic coordinates should be deposited in an appropriate database in time for the accession number to be included in the published article. In computational studies where the sequence information is unacceptable for inclusion in databases because of lack of experimental validation, the sequences must be published as an additional file with the article.
JLSB accept the manuscripts in PDF, Word or TeX/LaTeX formats; Word files are preferred especially those are prepared using EndNote®. However, our team will reformat the articles of non-EndNote users via EndNote in the alley proof stage, if accepted.
.
A JLSB reference style for EndNote may be found here.
How to install additional styles? Please click here.
How to turn on "Jumping" from a citation to the bibliography? Please click here.
.
We prefer Vancouver referencing style that is often used in medicine and the natural sciences. Uniform requirements for manuscripts submitted to Biomedical Journals, published by International Committee of Medical Journal Editors, includes a list with examples of references https://www.nlm.nih.gov/bsd/uniform_requirements.html in the Vancouver style. It is desirable to add a DOI (digital object identifier) number for each reference. If a DOI is lacking, please add a link to any online source of an article (Google Scholar is preferred).
- References should be numbered consecutively and cited in the text by number in square brackets like [1, 2] instead of parentheses (and not by author and date).
- References should not be formatted as footnotes. Avoid putting personal communications and unpublished observations as references, as much as possible. Papers that have been accepted for publication, but not yet specified for an issue should be cited as “to be published”. Papers that have been submitted for publication should be cited as “submitted for publication”.
- All references to publications made in the text should be presented in a list with their full bibliographical description. DOI number or the link of the article should be added to the end of each reference. All the cited papers in the text must be listed in References and all the papers in References must be cited in the text; To this end, we suggest you use our EndNote style.
- Names of authors and titles of journals published in non-latin alphabets should be transliterated in English, as much as possible. Please give the English citation first, followed by the original foreign-language citation.
- Capitalize only the first word in a paper title, except for proper nouns and element symbols.
- A sample of standard reference is "1th Author surname A, 2th Author surname B and 3th Author surname C. Article title should be regular and 9 or 10 pt. Journal of Life Science and Biomedicine or J. Life Sci. Biomed. 2020; Volume(Issue): 00-00." "DOI: https://dx.doi.org/10.00000/xxxx"; PMID: 000000; PMCID: PMC000000; "Google Scholar (https://scholar.google.com)"
- To find DOI of each reference easily, click here (https://apps.crossref.org/SimpleTextQuery); To find the reference with the acceptable format in PubMed click here (https://pubmed.ncbi.nlm.nih.gov/).
- From 2020, the journal titles should be full in references. The titles should not be italic.
- References with more than 6 authors should be listed, the first 6 authors followed by 'et al.'
- Use a space after authors’ initials.
- The color of references in the text of the article is blue. Example: [1, 2].
- At least 35% of the references of any submitted manuscript (for all types of article) should include scientific results published in the last five years.
-Examples (at the text- blue highlighted)
Smit [1] stated that ...; Smit and Janak [2] believe that …; Our results are in agreement with previous studies [3, 4] or study of Nurai et al. [5].
Acceptable Examples (at references section):
For Journals:
1. Chekani-Azar S, Gharib Mombeni E, Birhan M, and Yousefi M. CRISPR/Cas9 gene editing technology and its application to the coronavirus disease (COVID-19), a review. Journal of Life Science and Biomedicine. 2020; 10(1): 01-09; DOI: https://dx.doi.org/10.29252/scil.2020.jlsb1
2. Nazirov FG, Djuraeva NM, Vakhidova NT, Omonov OA and Salimov UR. Diagnostic informativity of the volume MDCT-angiography and MR-cholangiography in the pre- and intraoperative periods for the examination of donors of a liver fragment. J. Life Sci. Biomed., 2019; 9(6): 151-156. DOI: https://dx.doi.org/10.36380/scil.2019.jlsb24
3. Doll MA, Salazar-González RA, Bodduluri S and Hein DW. Arylamine N-acetyltransferase 2 genotype-dependent N-acetylation of isoniazid in cryopreserved human hepatocytes. Acta Pharm Sin B. 2017; 7(4):517-522. DOI: https://dx.doi.org/10.00000/xxxx; Link
4. Titomanlio L, Kavelaars A, Dalous J, Mani S, El Ghouzzi V, et al. Stem cell therapy for neonatal brain injury: Perspectives and challenges. Annals of neurology. 2011; 70 (5): 698-712. DOI: https://dx.doi.org/10.00000/xxxx; Link
5. Karen KS and Otto CM. Pregnancy in women with valvular heart disease. Heart. 2007 May; 93(5): 552–558. DOI: https://dx.doi.org/10.00000/xxxx; Link
For In press manuscripts (maximum 2)
Hasan V, Sri Widodo M and Semedi B. Oocyte Diamater Distribution and Fecundity of Javaen Barb (Systomus Orphoides) at the Start of Rainy Season in Lenteng River, East Java, Indonesia insurance. In press. 2015.
For symposia reports and abstracts
Cruz EM, Almatar S, Aludul EK and Al-Yaqout A. Preliminary Studies on the Performance and Feeding Behaviour of Silver Pomfret (Pampus argentens euphrasen) Fingerlings fed with Commercial Feed and Reared in Fibreglass Tanks. Asian Fisheries Society Manila, Philippine. 2000; 13: 191-199.
For Conference
Skinner J, Fleener B and Rinchiuso M. Examining the Relationship between Supervisors and Subordinate Feeling of Empowerment with LMX as A Possible Moderator. 24th Annual Conference for Industrial Organizational Behavior. 2003.
For Book
Russell, Findlay E. Snake Venom Poisoning, 163, Great Neck, NY: Scholium International. ISBN 0-87936-015-1. 1983.
For Web Site
Bhatti SA and Firkins JT. 2008. http://www.ohioline.osu.edu/sc1156_27.hmtl.
Nomenclature and Abbreviations
Nomenclature should follow that given in NCBI web page and Chemical Abstracts. Standard abbreviations are preferable. If a new abbreviation is used, it should be defined at its first usage. Abbreviations should be presented in one paragraph, in the format: "term: definition". Please separate the items by ";".
E.g. ANN: artificial neural network; CFS: closed form solution; ...
Abbreviations of units should conform with those shown below:
|
Decilitre
|
dl
|
Kilogram
|
kg
|
|
Milligram
|
mg
|
hours
|
h
|
|
Micrometer
|
mm
|
Minutes
|
min
|
|
Molar
|
mol/L
|
Mililitre
|
ml
|
|
Percent
|
%
|
.
|
Other abbreviations and symbols should follow the recommendations on units, symbols and abbreviations: in “A guide for Biological and Medical Editors and Authors (the Royal Society of Medicine London 1977). Papers that have not been published should be cited as “unpublished”. Papers that have been accepted for publication, but not yet specified for an issue should be cited as “to be published”. Papers that have been submitted for publication should be cited as “submitted for publication".
Formulae, numbers and symbols
- Typewritten formulae are preferred. Subscripts and superscripts are important. Check disparities between zero (0) and the letter 0, and between one (1) and the letter I.
- Describe all symbols immediately after the equation in which they are first used.
- For simple fractions, use the solidus (/), e.g. 10 /38.
- Equations should be presented into parentheses on the right-hand side, in tandem.
- Levels of statistical significance which can be used without further explanations are *P < 0.05, **P < 0.01, and ***P < 0.001.
- In the English articles, a decimal point should be used instead of a decimal comma.
- Use Symbol fonts for "±"; "≤" and "≥" (avoid underline).
- In chemical formulae, valence of ions should be given, e.g. Ca2+ and CO32-, not as Ca++ or CO3.
- Numbers up to 10 should be written in the text by words. Numbers above 1000 are recommended to be given as 10 powered x.
- Greek letters should be explained in the margins with their names as follows: Αα - alpha, Ββ - beta, Γγ - gamma, Δδ - delta, Εε - epsilon, Ζζ - zeta, Ηη - eta, Θθ - theta, Ιι - iota, Κκ - kappa, Λλ - lambda, Μμ - mu, Νν - nu, Ξξ - xi, Οο - omicron, Ππ - pi, Ρρ - rho, Σσ - sigma, Ττ - tau, Υυ - ipsilon, Φφ - phi, Χχ - chi, Ψψ - psi, Ωω - omega.Please avoid using math equations in Word whenever possible, as they have to be replaced by images in xml full text.
.
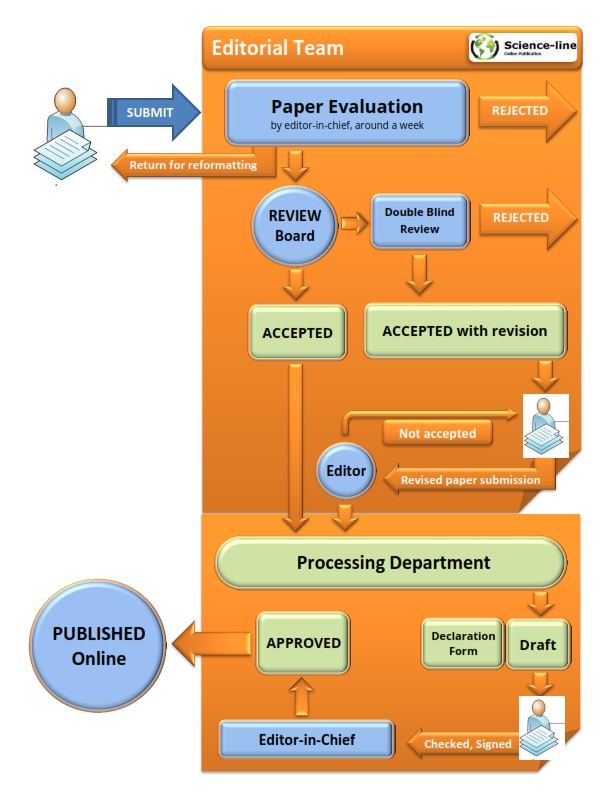
Firstly, all manuscripts will be checked by one of the plagiarism finding tools (iThenticate, PlagScan and or Docol©c). A double-blind reviewing model is used by JLSB for non-plagiarized papers. The manuscript is edited and reviewed by the English language editor and three reviewers selected by section editor of JLSB respectively. Also, a reviewer result form is filled by reviewer to guide authors. Possible decisions are: accept as is, minor revision, major revision, or reject. The estimated time from submission to first decision is 3.5 weeks and the estimated time from submission to final decision is 6.4 weeks. The estimated time for final publication of accepted manuscript is 5 weeks.
To submit a revision please click here, fill out the form, and mark "Revised", mention the article code (for example JLSB-1105), attach the revision (MS word) and continue submission. After review and editing the article, a final formatted proof is sent to the corresponding author once again to apply all suggested corrections during the article process. The editor who received the final revisions from the corresponding authors shall not be hold responsible for any mistakes shown in the final publication. Manuscripts with significant results are typically reviewed and published at the highest priority.
Plagiarism
There is an instant policy towards plagiarism (including self-plagiarism) in our journal. Manuscripts are screened for plagiarism by iThenticate, before or during publication, and if found they will be rejected at any stage of processing.
Declaration
After manuscript accepted for publication, a declaration form will be sent to the corresponding author who that is responsible to coauthors' agreements to publication of submitted work in JLSB after any amendments arising from the peer review.
Date of issue
The journal will be issued on 25th of March, June, September and December, each year.
Publication charges
The publication costs are covered through article processing charges (APCs) and no submission or any other fees are required for the publication of the accepted article. There is a modest APC of 100 Euro(€) editor fee for the processing of each primary accepted paper (1000-4000 words) to encourage high-quality submissions. APCs will be considered for articles that successfully pass the peer-review process and are ready for publication. A surcharge will be placed on any article that is over 4000 words in length to cover the considerable additional processing costs. Payment can be made by credit card, bank transfer, money order or check. Instruction for payment is sent during publication process as soon as manuscript is accepted. Meanwhile, this journal encourages the academic institutions in low-income countries to publish high quality scientific results, free of charges.
| WORD COUNT | PRICE* |
| 1000-4000 words | €100 |
| over 4000 words |
€120 |
* The prices are valid until 30th December 2026.
In response to the time-sensitive nature of academic publishing, JLSB recognizes the various deadlines authors often face, such as those related to promotion and tenure, thesis defense, grant proposals, research fund expenditures, subsequent paper publications, and the urgency of disseminating critical findings. To support authors facing such deadlines, JLSB offers a fast-track review process. However, the journal imposes no processing fee for submitted manuscripts in a fast-track review process. In the event of rejection, authors are exempt from any fees in the fast-track review process. Upon acceptance, authors are required to pay the publication fee (calculated based on the total word count) plus an additional 100 Euros when an invoice and the declaration form are sent to them. Authors submitting a manuscript to JLSB will receive an email explaining the conditions for the fast-track review process within a working day of submission. They can initiate the process by confirming the conditions of the fast-track review process and contacting the journal via email. It is essential to emphasize that the request for a fast-track review does not affect the editorial decision-making process, maintaining the integrity and thoroughness of our evaluation standards.
The Waiver policy
The publication fee will be waived for invited authors, authors of hot papers, and corresponding authors who are editorial board members of the Journal of Life Science and Biomedicine. The Journal will consider requests to waive the fee for cases of financial hardship (for high quality manuscripts and upon acceptance for publication). Requests for waiver of the publication fee must be submitted via individual cover letter by the corresponding author and cosigned by an appropriate institutional official to verify that no institutional or grant funds are available for the payment of the fee. Letters including the manuscript title and manuscript ID number should be sent to: This email address is being protected from spambots. You need JavaScript enabled to view it.. It is expected that waiver requests will be processed and authors will be notified within two business day.
Publishing model and Open Access policy
The Journal of Life Science and Biomedicine is an gold open access journal which means that all content (research articles, review articles, case reports, educational papers) is freely and permanently accessible to everyone, immediately after publication. Most permission barriers are removed from the journal's webpage (https://jlsb.science-line.com/), and the copyright for the article is retained by the authors. Users are allowed to read, download, copy, distribute, print, search, or link to the full texts of the articles, or use them for any other lawful purpose, without asking prior permission from the publisher or the author. This is in accordance with the BOAI definition of Open Access. For additional information please see Policies and Publication Ethics.
.
Scienceline Language Editing Services
We suggest that authors whose first language is not English have their manuscripts checked by a native English speaker before submission. This is optional, but will help to ensure that any submissions that reach peer review can be judged exclusively on academic merit. We offer a Scienceline service, and suggest that authors contact as appropriate. Please note that use of language editing services is voluntary, and at the author’s own expense. Use of these services does not guarantee that the manuscript will be accepted for publication, nor does it restrict the author to submitting to a Scienceline journals. You can send the article/s to the following Emails: This email address is being protected from spambots. You need JavaScript enabled to view it. ; This email address is being protected from spambots. You need JavaScript enabled to view it.
For more information about editing services please visit here.
Paper Submission Flow
 Submission Preparation Checklist
Submission Preparation Checklist
Authors are required to check off their submission's compliance with all of the following items, and submissions may be returned to authors that do not adhere to the following guidelines.
The submission has not been previously published, nor is it before another journal for consideration (or an explanation has been provided in Comments to the Editor).
The submission file is in Microsoft Word, RTF, or PDF document file format.
Where available, URLs for the references have been provided.
The text is single-spaced; uses a 12-point font; and all illustrations, figures, and tables are placed within the text at the appropriate points, rather than at the end.
The text adheres to the stylistic and bibliographic requirements outlined in the Author Guidelines.
(Revised on September 01, 2025)
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Publisher Item Identifier
A Publisher Item Identifier (PII) is a standard identifier number system which identifies a published document. It was created in 1996, agreed and developed by many of international societies like by the American Chemical Society, the American Institute of Physics, the American Physical Society, Elsevier Science, and the IEEE. Although, PII has currently been included in Digital Object Identifier (DOI)® system (as a syntaxe in the DOI suffix) for Crossref publishers using De facto identifier method, but many of publishers like AMS, still rely on original, facil and free of charge method of creating the Article Unique Identifiers like PII.
Digital Object Identifier (DOI): A name (not a location) for an entity on digital networks. DOI names are used to provide current information, including where they (or information about them) can be found on the Internet.
Publisher Item Identifier (PII): Alphanumeric string of characters that uniquely identifies the article and can be used for future cataloging, searching, and electronic retrieval (Free of Charge). An article explaining the PII numbering system can be found on: http://en.wikipedia.org/wiki/Publisher_Item_Identifier.
-
- 2019 (Volume 9)
- Article 01: S225199391900001-9
- Article 02: S225199391900002-9
- Article 03: S225199391900003-9
- Article 04: S225199391900004-9
- Article 05: S225199391900005-9
- Article 06: S225199391900006-9
- Article 07: S225199391900007-9
- Article 08: S225199391900008-9
- Article 09: S225199391900009-9
- Article 10: S225199391900010-9
- Article 11: S225199391900011-9
- Article 12: S225199391900012-9
- Article 13: S225199391900013-9
- Article 14: S225199391900014-9
- Article 15: S225199391900015-9
- Article 16: S225199391900016-9
- Article 17: S225199391900017-9
- Article 18: S225199391900018-9
- Article 19: S225199391900019-9
- Article 20: S225199391900020-9
- Article 21: S225199391900021-9
- Article 22: S225199391900022-9
- Article 23: S225199391900023-9
- Article 24: S225199391900024-9
- Article 25: S225199391900025-9
- Article 26: S225199391900026-9
- Article 27: S225199391900027-9
- 2020 (Volume 10)
- Article 01: S225199392000001-10
- Article 02: S225199392000002-10
- Article 03: S225199392000003-10
- Article 04: S225199392000004-10
- Article 05: S225199392000005-10
- Article 06: S225199392000006-10
- Article 07: S225199392000007-10
- Article 08: S225199392000008-10
- Article 09: S225199392000009-10
- Article 10: S225199392000010-10
- Article 11: S225199391600031-6
- Article 12: S225199391600032-6
- Article 33: S225199391600033-6
- Article 34: S225199391600034-6
- Article 35: S225199391600035-6
- Article 36: S225199391600036-6
- Article 37: S225199391600037-6
- Article 38: S225199391600038-6
- Article 39: S225199391600039-6
- Article 31: S225199391600031-6
- Article 32: S225199391600032-6
- Article 33: S225199391600033-6
- Article 34: S225199391600034-6
- Article 35: S225199391600035-6
- Article 36: S225199391600036-6
- Article 37: S225199391600037-6
- Article 38: S225199391600038-6
- 2021 (Volume 11)
- Article 01: S225199392100001-11
- Article 02: S225199392100002-11
- Article 03: S225199392100003-11
- Article 04: S225199392100004-11
- Article 05: S225199392100005-11
- Article 06: S225199392100006-11
- Article 07: S225199392100007-11
- Article 08: S225199392100008-11
- Article 09: S225199392100009-11
- Article 10: S225199392100010-11
- Article 11: S225199392100011-11
- Article 12: S225199392100012-11
- Article 13: S225199392100013-11
- Article 14: S225199392100014-11
- Article 35: S225199391600035-6
- Article 36: S225199391600036-6
- Article 37: S225199391600037-6
- Article 38: S225199391600038-6
- Article 39: S225199391600039-6
- Article 31: S225199391600031-6
- Article 32: S225199391600032-6
- Article 33: S225199391600033-6
- Article 34: S225199391600034-6
- Article 35: S225199391600035-6
- Article 36: S225199391600036-6
- Article 37: S225199391600037-6
- Article 38: S225199391600038-6
-
- 2016 (Volume 6)
- Article 01: S225199391600001-6
- Article 02: S225199391600002-6
- Article 03: S225199391600003-6
- Article 04: S225199391600004-6
- Article 05: S225199391600005-6
- Article 06: S225199391600006-6
- Article 07: S225199391600007-6
- Article 08: S225199391600008-6
- Article 09: S225199391600009-6
- Article 10: S225199391600010-6
- Article 11: S225199391600011-6
- Article 12: S225199391600012-6
- Article 13: S225199391600013-6
- Article 14: S225199391600014-6
- Article 15: S225199391600015-6
- Article 16: S225199391600016-6
- Article 17: S225199391600017-6
- Article 18: S225199391600018-6
- Article 19: S225199391600019-6
- Article 20: S225199391600020-6
- Article 21: S225199391600021-6
- 2017 (Volume 7)
- Article 01: S225199391700001-7
- Article 02: S225199391700002-7
- Article 03: S225199391700003-7
- Article 04: S225199391700004-7
- Article 05: S225199391700005-7
- Article 06: S225199391700006-7
- Article 07: S225199391700007-7
- Article 08: S225199391700008-7
- Article 09: S225199391700009-7
- Article 10: S225199391700010-7
- Article 11: S225199391700011-7
- Article 12: S225199391700012-7
- Article 13: S225199391700013-7
- Article 14: S225199391700014-7
- Article 15: S225199391700015-7
- Article 36: S225199391600036-6
- Article 37: S225199391600037-6
- Article 38: S225199391600038-6
- Article 39: S225199391600039-6
- Article 36: S225199391600036-6
- Article 40: S225199391600040-6
- 2018 (Volume 8)
- Article 01: S225199391800001-8
- Article 02: S225199391800002-8
- Article 03: S225199391800003-8
- Article 04: S225199391800004-8
- Article 05: S225199391800005-8
- Article 06: S225199391800006-8
- Article 07: S225199391800007-8
- Article 08: S225199391800008-8
- Article 09: S225199391800009-8
- Article 10: S225199391800010-8
- Article 11: S225199391800011-8
- Article 12: S225199391800012-8
- Article 13: S225199391800013-8
- Article 14: S225199391800014-8
- Article 15: S225199391800015-8
- Article 36: S225199391600036-6
- Article 37: S225199391600037-6
- Article 38: S225199391600038-6
- Article 39: S225199391600039-6
- Article 36: S225199391600036-6
- Article 40: S225199391600040-6
2015 (Volume 5)
-
- Article 01: S225199391500001-5
- Article 02: S225199391500002-5
- Article 03: S225199391500003-5
- Article 04: S225199391500004-5
- Article 05: S225199391500005-5
- Article 06: S225199391500006-5
- Article 07: S225199391500007-5
- Article 08: S225199391500008-5
- Article 09: S225199391500009-5
- Article 10: S225199391500010-5
- Article 11: S225199391500011-5
- Article 12: S225199391500012-5
- Article 13: S225199391500013-5
- Article 14: S225199391500014-5
- Article 15: S225199391500015-5
- Article 16: S225199391500016-5
- Article 17: S225199391500017-5
- Article 18: S225199391500018-5
- Article 19: S225199391500019-5
- Article 20: S225199391500020-5
- Article 21: S225199391500021-5
- Article 22: S225199391500022-5
- Article 23: S225199391500023-5
- Article 24: S225199391500024-5
- Article 25: S225199391500025-5
- Article 26: S225199391500026-5
- Article 27: S225199391500027-5
- Article 28: S225199391500028-5
- Article 29: S225199391500029-5
- Article 30: S225199391500030-5
- Article 31: S225199391500031-5
- Article 32: S225199391500032-5
- Article 33: S225199391500033-5
- Article 34: S225199391500034-5
- Article 35: S225199391500035-5
- Article 36: S225199391200036-5
- Article 37: S225199391200037-5
- Article 38: S225199391200038-5
- Article 39: S225199391200039-2
- Article 40: S225199391200040-2
.
2014 (Volume 4)
|
|
|
|
|
|
|
|
2013 (Volume 3)
|
|
|
|
2012 (Volume 2)
|
|
|
2011 (Volume 1)
|
![]() Submit to the Journal through one of the following ways:
Submit to the Journal through one of the following ways:
.
1- JLSB Online Submission Form (if you can't see the Submission Form below, use a VPN)
2- SCIENCELINE Online Submission System ![]()
.
Simultaneous and Multiple Submissions
We do consider only online submissions, but not multiple submissions, so please send only one manuscript at a time, either by JLSB or Scienceline Submission Forms. Do not send a second submission until you have heard about the first. Simultaneous submissions to other journals are also not allowed. Please visit JLSB instructions prior to submission.
CHECKLIST prior to initial submission: Authors must ensure that they have available the following items, otherwise submissions may be returned.
- The complete manuscript (.doc/.docx) including title page and abstract, and also high-resolution graphs, figures, schemes, equations and, if complex, tables at the end of main text. Please make sure that each figure etc is clearly labelled with its number.
- Between two and six keywords or short relevant phrases
- The text adheres to the bibliographic requirements outlined in the Author Guidelines. Where available, URLs and DOIs (find here) for the references should be provided.
- Supplementary data should be in a file(s) separate from the main manuscript file (send via email, if any).
- A short Cover Letter to the editor, either as a file to submit by email or via the submission system.
- The submission has not been previously published, and have not submitted in another journal concurrently.