Previous issue | Next issue | Archive
| Comprehensive overview, molecular epidemiology and antimicrobial resistance in Non-typhoid Salmonella |
Comprehensive overview, molecular epidemiology and antimicrobial resistance in Non-typhoid Salmonella
Alemnew M, Gelaw A, Nibret K, Getu A, and Berhane N.
J. Life Sci. Biomed., 13 (2): 25-34, 2023; pii:S225199392300004-13
DOI: https://dx.doi.org/10.54203/jlsb.2023.4
Abstract
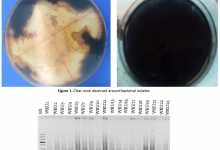
Non-typhoid Salmonella (NTS) is a major public health problem affecting both humans and animals in many countries and being an important public health problem worldwide. It is a leading bacterial cause of diarrheal disease in humans, leading to gastroenteritis and deaths. It is a major agent of food-borne outbreaks as well as individual cases, especially in developing countries. Many virulence genes of Salmonella enterica are organized on SPIs (Salmonella pathogenicity islands). Twenty three SPIs are identified in genus Salmonella, which are required for adhesion, invasion, intra-cellular survival, and replication. Enhanced surveillance, improved food safety and responsible antibiotic use are crucial for preventing the spread of NTS. This review offers detailed information on NTS in an inclusive manner rather than fragmented approach. It also focuses on human NTS infections, unlike most research, which is conducted on animals.
Keywords: Antimicrobial resistance, Antimicrobial resistance gene, Salmonella, Non-typhoid Salmonella (NTS), Salmonella pathogenicity islands (SPIs)
[Full text-PDF] [ePub] [Export citation from ePrint] [How to Cite]
| Therapeutic aspects of dietary fibre and glycemic index: a brief review |
Prevention of alloimmunization in patients with sickle cell disease in Chad
Djimadoum M, Nadlaou B, Kaboro M, Christian D, Alio HM, Kimassoum R.
J. Life Sci. Biomed., 13(2): 35-41, 2023; pii:S225199392300005-13
DOI: https://dx.doi.org/10.54203/jlsb.2023.5
Abstract
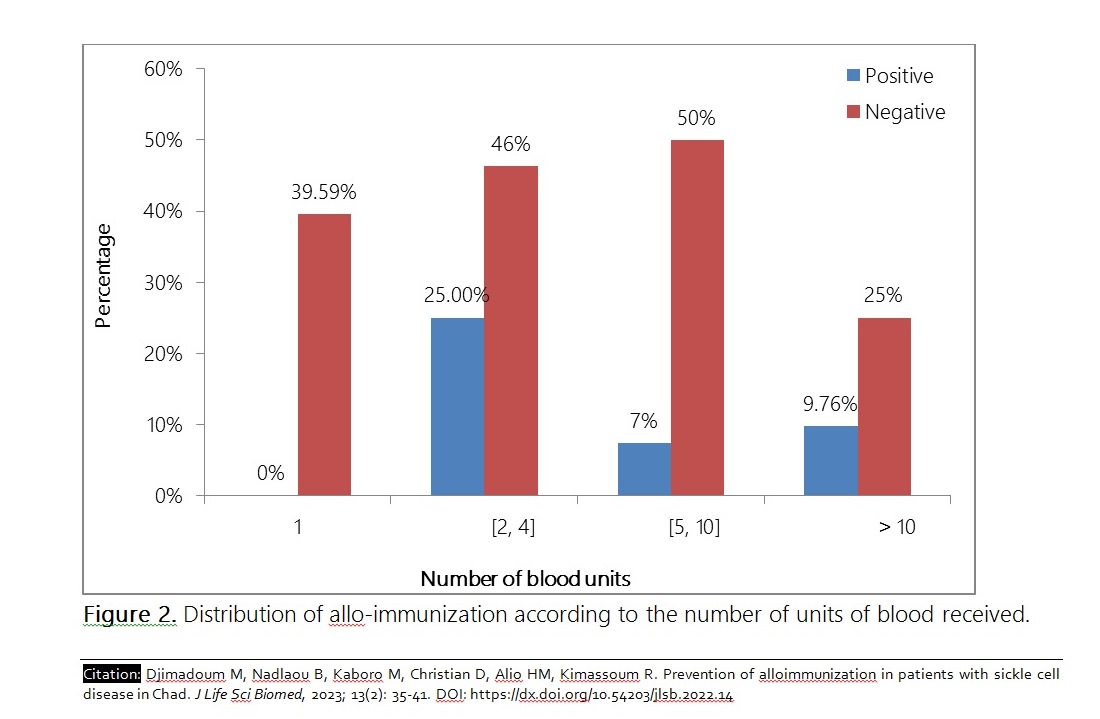
The objective of this study was to contribute to the prevention of anti-erythrocyte alloimmunization in patients with sickle cell disease (DS) in Chad. This was a descriptive and analytical cross-sectional study from December 2021 to June 2022 in sickle cell patients regularly followed in two University Hospital Centers (CHU) in N'Djamena. The search for irregular antibodies was carried out by a combination of three techniques: indirect anti-globulin test (Coombs), enzymatic test (Papain) and the test in a low ionic strength medium, in a partner laboratory of the National Blood Transfusion Center in France. The study included 57 sickle cell patients, 45 of whom (78.9%) were actually transfused. Sickle cell patients accounted for 84.2% of cases. The sex ratio Male/Female (M/F) was 1.03. The average age of the transfused was 9.3 ± 5.4 years. Four of the transfused patients (8.8%) had produced 7 antibodies including 85.7% anti-rhesus and 14.3% anti-Kell. Age, sex and number of blood units were associated with antibody production with probabilities of 0.046, 0.041 and 0.035, respectively. In view of the results obtained from this study, we recommend the application of the blood transfusion rules and procedures in force in Chad.
Keywords: Allo-immunization, sickle cell disease, prevention, Chad
[Full text-PDF] [ePub] [Export citation from ePrint] [How to Cite]
| Evaluating the effectiveness of isolated fungi against Fall armyworm (Spodoptera frugiperda) |
A proposed new method of hemostasis for erosive-hemorrhagic complications of the trachea and bronchi
Azizova GM, Ibragimov SK, Arifjanov AS, Ibadov RA.
J. Life Sci. Biomed., 13(2): 42-47, 2023; pii:S225199392300006-13
DOI: https://dx.doi.org/10.54203/jlsb.2023.6
Abstract
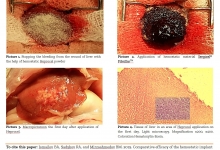
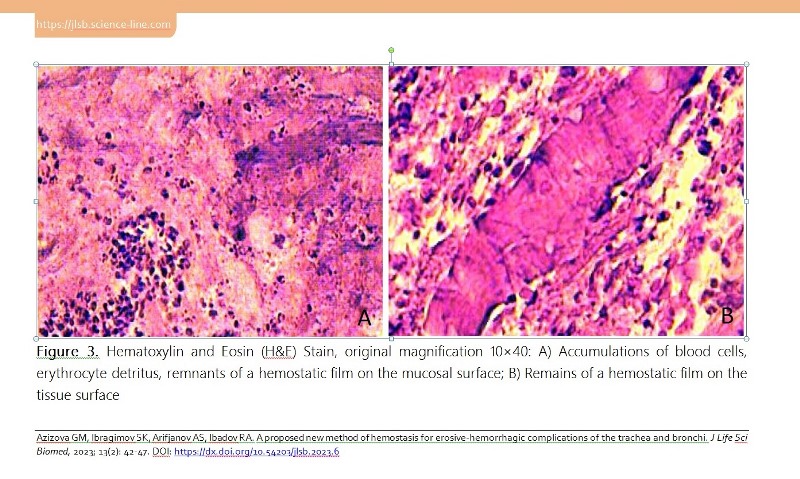
Ventilator-associated complications are the most frequent ones that prevent the recovery of intensive care patients. This study aimed to evaluate the effectiveness of the endoscopic treatment of acute hemorrhagic lesions of the tracheobronchial tree in patients with ventilator-associated tracheobronchitis. A total of 5 patients (22.4±3.3 mean age, 18 to 36 years) with prolonged mechanical ventilation (>48 hours) in the resuscitation and intensive care unit were monitored. The diagnosis of ventilator-associated tracheobronchitis was established on the basis of clinical signs, radiological and microbiological criteria. Fine-dispersed powder with hemostatic properties (HEMOBEN) was used as a hemostatic agent when applying the method. A morphometric study noted that ventilator-associated tracheobronchitis is accompanied by a pronounced lymphoid-neutrophilic infiltration of the tracheal and bronchial mucosa (signs of diffuse catarrhal inflammation of varying degrees and erosive deforming endobronchitis are verified), and in a complicated course with the presence of a purulent-inflammatory and erosive-hemorrhagic component with its vascularization and metaplastic changes and violations of the integrity of the epithelial lining also leads to a violation of the integrity of tissues due to destruction and necrosis. Hence, a method has been developed to stop bleeding in case of mucosal damage by local application of HEMOBEN, which allows us to quickly cover the bleeding surface with an adequate hemostatic effect.
Keywords: Ventilator-associated tracheobronchitis, prolonged mechanical ventilation, clinic, endoscopic treatment, pathomorphological changes
[Full text-PDF] [ePub] [Export citation from ePrint] [How to Cite]
Previous issue | Next issue | Archive
![]() This work is licensed under a Creative Commons Attribution 4.0 International License (CC BY 4.0)
This work is licensed under a Creative Commons Attribution 4.0 International License (CC BY 4.0)