Previous issue | Next issue | Archive
| Review on the biosafety measures for the prevention and control of SARS-CoV-2 infections in healthcare settings |
Review COVID-19
Review on the biosafety measures for the prevention and control of SARS-CoV-2 infections in healthcare settings
Alemnew M, Andualem B, Gelaw A, Kassa E, Nibret K, and Getu A.
 J. Life Sci. Biomed., 14(2): 30-37, 2024; pii:S225199392300003-14
J. Life Sci. Biomed., 14(2): 30-37, 2024; pii:S225199392300003-14
DOI: https://dx.doi.org/10.54203/jlsb.2024.3
Abstract
The novel corona virus, Severe Acute Respiratory Syndrome Corona Virus 2 (SARS‐CoV‐2) is a deadly respiratory disease. The major causes of the rise of new variants of SARS-CoV-2 are genetic mutations and recombination. The club-shaped spike (S) projections of glycoprotein on the virus's envelope has interaction with the host cell’s ACE2 (Angiotensin-Converting Enzyme 2) receptors to enable the entrance of infectious virus particles. With-Corona’ is possible only when antiviral and vaccination are available. Disease prevention and control is based on new biosafety technologies and guidelines which improve ways to safely handle microbiological material. Corona pandemic is a serious biosafety event. Thus, biosafety is being valued worldwide and applied in medical field. This review presents the current biosafety standards of the COVID-19 pandemic in a comprehensive manner from a fragmented or wide variety of biosafety guidelines and articles available.
Keywords:
[Full text-PDF] [ePub] [Export citation from ePrint] [How to Cite]
| Morphometric study of peripheral blood in assessing the appropriateness of colloid use after mitral valve replacement |
Research Paper
Morphometric study of peripheral blood in assessing the appropriateness of colloid use after mitral valve replacement
Ibadov RA, Ergashev SP, and Ibragimov SK.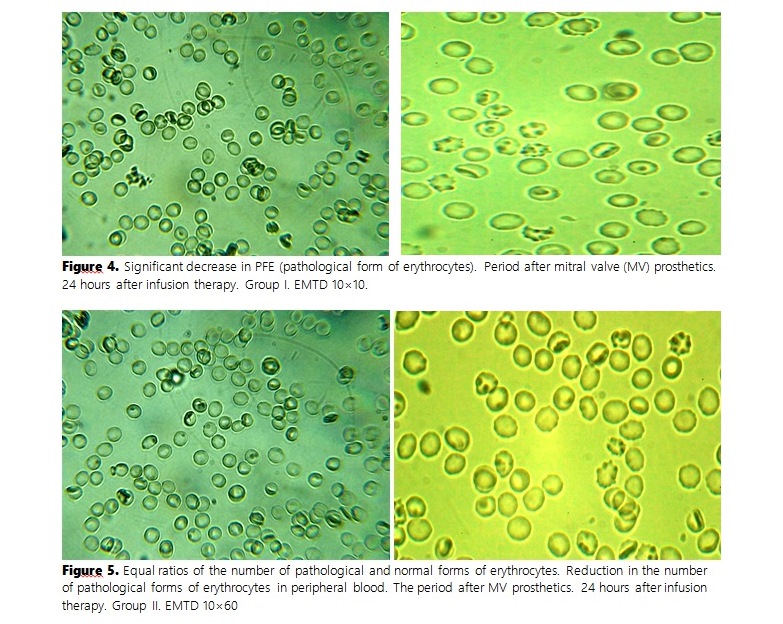 J. Life Sci. Biomed., 14(2): 38-44, 2024; pii:S225199392300004-14
J. Life Sci. Biomed., 14(2): 38-44, 2024; pii:S225199392300004-14
DOI: https://dx.doi.org/10.54203/jlsb.2024.4
Abstract
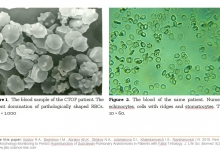
The study aimed to evaluate the morphological changes in erythrocytes following the administration of various solutions. Patients who underwent mitral valve (MV) replacement were divided into three groups: the first group received Hydroxyethylcellulose (HEC), the second received gelatin solution (4% succinylated gelatin solution), and the third received 0.9% sodium chloride solution. Blood samples were collected at 90 minutes, 24, and 48 hours post-resuscitation (ICU), and before discharge. Healthy patients' blood served as the control. The express technique of the "thick drop" (EMTD) was used to assess erythrocyte shape shifts, measuring the ratio of normal and pathological forms within 10-15 minutes using a DN-300M microscope. Before using the solutions, the pathological forms of erythrocytes were significantly higher in all groups compared to the control group. The control group had over 89.8% discocytes, while the number significantly decreased in MV patients pre-operation. Post-treatment with HEC and Gelatin solution, the number of discocytes increased slightly, whereas the third group (sodium chloride) showed no significant change. The number of echinocytes and stomatocytes varied across the groups, with the third group showing minimal improvement. The findings indicated that HEC and gelatin solution improved the ratio of discocytes to pathological forms better than sodium chloride. The most promising plasma-stimulating agents for surgical patients, particularly those with heart disease, are colloids and balanced crystalloid solutions. Their composition closely mimics blood plasma, providing optimal hemodynamic stability and tissue perfusion, thus supporting better postoperative recovery and critical care management.
Keywords: Erythrocyte Morphology, Mitral Valve Replacement, Colloid Solutions, Crystalloid Solutions, Postoperative Hemodynamics
[Full text-PDF] [ePub] [Export citation from ePrint] [How to Cite]
Previous issue | Next issue | Archive
![]() This work is licensed under a Creative Commons Attribution 4.0 International License (CC BY 4.0)
This work is licensed under a Creative Commons Attribution 4.0 International License (CC BY 4.0)